Abstract
Background: Upon first-line treatment failure, patients (pts) with DLBCL may be candidates for HSCT if they respond to salvage chemotherapy. Pts unable to undergo HSCT have poor outcomes with conventional therapies. In recent years, CAR T cell therapy, pola, and tafa demonstrated efficacy in clinical trials as new treatment options for R/R DLBCL. This study describes demographics, clinical characteristics, treatment patterns, and health care costs among pts with R/R DLBCL in the United States who received CAR T cell therapies, pola, or tafa in the real-world clinical setting.
Methods: A retrospective observational study was performed in adults with R/R DLBCL from 01/01/2009 to 12/31/2021 using the PharMetrics Plus claims database. The index date was defined as first date of CAR T cell therapy, pola, or tafa infusion during the index period from 10/01/2017 to 11/30/2021. CAR T cell therapies included lisocabtagene maraleucel (liso-cel), axicabtagene ciloleucel (axi-cel), tisagenlecleucel, or unspecified product. Pts ≥ 18 y of age diagnosed with DLBCL who received 1 of the treatments of interest during the index period were eligible. Pts with < 1 month of continuous pharmaceutical and medical benefit enrollment on, before (CAR T cell therapy cohort only), or after (pola and tafa cohorts only) the index date were excluded. All costs were adjusted to 2021 US dollars.
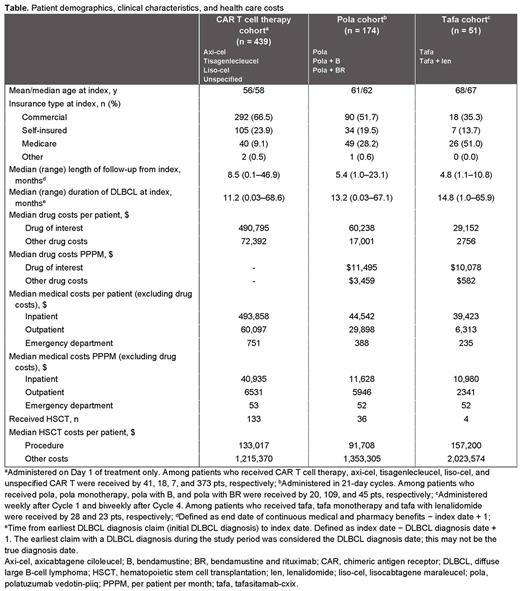
Results: Of 47,809 pts in the database diagnosed with DLBCL during the study period, 439 received CAR T cell therapies, 174 received pola (including pola monotherapy [n = 20], pola with bendamustine [n = 109], and pola with bendamustine and rituximab [n = 45]), and 51 received tafa (including tafa monotherapy [n = 28] and tafa in combination with lenalidomide [n = 23]). The median follow-up time was 8.5 months, 5.4 months, and 4.8 months, respectively. The mean age at index date for the CAR T cell therapy, pola, and tafa cohorts was 56, 61, and 68 y, respectively. The median duration of DLBCL at the index date for the 3 cohorts was 11.2, 13.2, and 14.8 months, respectively. HSCT was received during any time in the study period by 133 (30.3%), 36 (20.7%), and 4 (7.8%) pts in the CAR T cell therapy, pola, and tafa cohorts, respectively. Pts in the CAR T cell therapy cohort received 1 of the following therapies at the index date: axi-cel (41, 9.3%), tisagenlecleucel (18, 4.1%), liso-cel (7, 1.6%), or unspecified/unknown CAR T cell therapy (373, 85.0%). In the same cohort, pola and tafa were used before CAR T cell therapy in 28 (6.4%) and 1 (0.2%) pts, respectively, and after CAR T cell therapy in 38 (8.7%) and 11 (2.5%) pts, respectively. In the pola cohort, CAR T cell therapy and tafa were used before pola in 38 (21.8%) and 3 (1.7%) pts, respectively, and after pola in 28 (16.1%) and 19 (10.9%) pts, respectively. In the tafa cohort, CAR T cell therapy and pola were used before tafa in 11 (18.6%) and 19 (32.2%) pts, respectively, and after tafa in 1 (1.7%) and 3 (5.1%) pts, respectively. The median time to index treatment discontinuation was 1.0 month (95% CI, 0.7‒1.4) and 2.7 months (95% CI, 1.4‒3.6) for the pola and tafa cohorts, respectively. The median drug cost of the index drug per pt was highest for the CAR T cell therapy cohort compared with the pola and tafa cohorts (Table) owing to 1-time infusion/drug acquisition of CAR T cell therapy and short duration of treatment with pola and tafa. The median drug cost in the CAR T cell therapy cohort was $564,031, $556,394, $459,318, and $473,882 for axi-cel, tisagenlecleucel, liso-cel, and unspecified CAR T cell therapy, respectively. The median per pt per month drug cost of the index drug was $11,495 for pola and $10,078 for tafa (Table). In pts undergoing HSCT, median costs of the HSCT procedure and other costs (excluding procedure costs) were the highest in the tafa cohort ($157,200 and $2,023,574, respectively).
Conclusions: Despite recent advances in the treatment of DLBCL, our study found that use of novel pharmacological agents remains limited. Most patients treated with tafa and pola discontinued their treatments within 1-3 months. CAR T cell therapy was associated with the highest cost owing to cost of drug acquisition. Further research is needed to understand patient outcomes and the economic implications of treatment selection in the routine clinical setting.
Disclosures
Liu:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Subbiah:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Gu:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Le:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company.
Author notes
Asterisk with author names denotes non-ASH members.